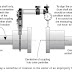
In automobile catalytic converters, the surface conditions of the precious metals—the catalyst materials—have a large effect on their ability to clean emissions. Conventionally, precious metal particles are adhered to a base material. However, heat from the exhaust gas causes the particles to collect together and agglomerate to form larger particles. This reduces the surface area of the precious metals and deteriorates their performance as catalysts. To counter this effect, large amounts of precious metals must be used in conventional catalytic converters. As an alternative, Mazda takes advantage of single nanotechnology to realize a unique and new catalyst structure in which precious metal particles are individually embedded into the base material.
The new catalyst has two main features.
1. It inhibits the thermal deterioration caused by the agglomeration of precious metal particles
2. It offers a significant improvement in oxygen absorption and release rates for enhanced emissions cleaning
With these features, the amount of precious metals needed to ensure the same level of effectiveness is reduced by 70 to 90 percent compared to previous products. At the same time, the performance of the catalytic converter is almost unaffected by harsh driving styles.
This technology can significantly reduce the amounts of expensive precious metals such as platinum, palladium and rhodium needed for three-way catalysts to effectively clean exhaust emissions from gasoline engines.