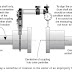
Crystalline Materials:
A crystalline material is one in which the atoms are situated in a repeating (or) periodic array over large atomic distances.

Non Crystalline Materials:
Materials that do not crystallize are called non-crystalline (or) Amorphous materials
Space Lattice:
Lattice is the regular geometrical arrangement of points in crystal space.

The atoms arrange themselves in distinct pattern in space is called a Space Lattice.
Atoms in crystalline materials are arranged in a regular 3 – Dimensional repeating pattern known as Lattice Structure.
They are divided by network of lines in to equal volumes, the points of intersection are known as Lattice Points.
Unit Cell:

It is the smallest portion of the lattice which repeated in all directions.
3D visualization of 14 Space Lattices are known as Bravai’s Space Lattice.
If a unit cell contains lattice points only at it’s corners, then it is called Primitive Unit Cell (or) Simple Unit Cell.
Three edge length x,y, & z and three interaxial angles α, β, & γ are termed as Lattice Parameters.
Crystal System:
It is a scheme by which crystal structures are classified according to unit cell geometry.
Types of Crystal Systems:
Cubic
Tetragonal
Hexagonal
Orthorhombic
Rhombohedral
Monoclinic
Triclinic
Crystal Systems
Simple Crystal Structure:
Body Centered Cubic Structure (BCC)
Unit cell contains 2 atoms
Lattice Constant a= 4r / √3, where r is atomic radius
Atomic packing factor APF = 0.68
Metals are Vanadium, Molybdenum, Titanium, Tungsten
Face Centered Cubic (FCC)
Unit cell contains 4 atoms
Lattice Constant a= 4r / √2, where r is atomic radius
Atomic packing factor APF = 0.72
FCC structures can be plastic deformed at severe rates
Metals are Copper, Aluminum, Phosphorous, Nickel, Cobalt etc
Unit cell contains 3 atoms
Axial ratio c/a, where ‘c’ is Distance between base planes, ‘a’ is Width of Hexagon
Axial Ratio varies from 1.58 for Beryllium to 1.88 for Cadmium (Therefore a=2.9787, c=5.617)
Atomic packing factor APF = 0.74
Metals are Zinc, Cadmium, Beryllium, Magnesium etc
Crystallographic Planes and Directions
The Layers of atoms in the planes along which atoms are arranged is known as “Atomic” (or) “Crystallographic planes”.
Miller Indices:
Miller Indices is a system of notation that denotes the orientation of the faces of a crystal and the planes and directions of atoms within that crystal.
Miller Indices for Planes:
1. The (110) surface
Intercepts : a , a , ∞
Fractional intercepts : 1 , 1 , ∞
Miller Indices : (110)
2. The (111) surface
Intercepts : a , a , a
Fractional intercepts : 1 , 1 , 1
Miller Indices : (111)
The (100), (110) and (111) surfaces considered above are the so-called low index surfaces of a cubic crystal system.
3. The (210) surface
Intercepts : ½ a , a , ∞
Fractional intercepts : ½ , 1 , ∞
Miller Indices : (210)